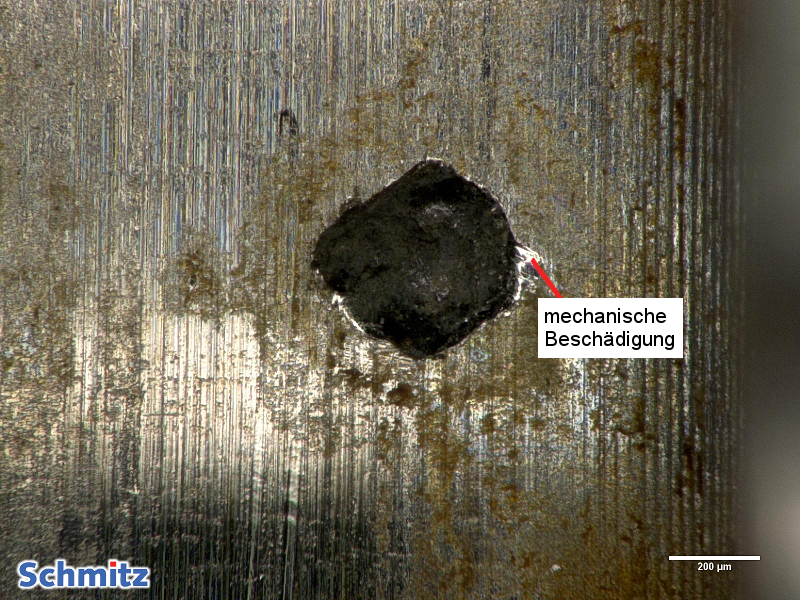
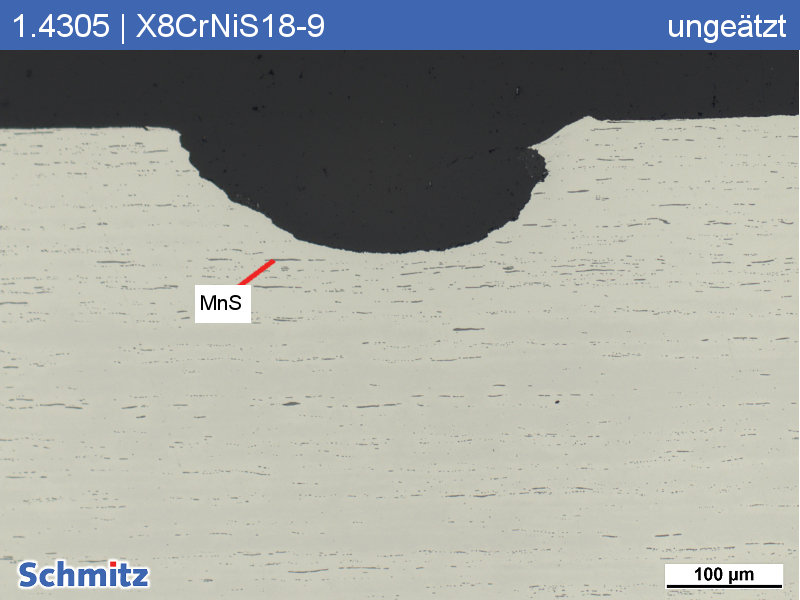
Pitting corrosion in 1.4305 | X8CrNiS18-9 | AISI 303 | S30300 after 24h use in “stale water”
| Numeric designation | 1.4305 | AISI 303 | S30300 |
|---|---|
| Chemical designation | X8CrNiS18-9 |
| State | Pitting corrosion |
| Etching | V2A-Beize @ 70 °C |
Cause of damage and corrosion mechanism:
The manganese sulfides (typical of this material) locally interfere with passivation of the surface. Locally, mechanical damage is also present in the area of corrosion damage. In addition to MnS, mechanical damage also injured the passive layer protecting against corrosive attacks. Using EDS analysis, a concentration of the element chlorine was detected, which caused the pitting corrosion. The resistance to chlorine is max. 200 mg/L at room temperature. To make matters worse, the small hole diameter created means that little oxygen gets in, preventing repassivation. In addition, since the oxygen content outside the hole is much greater than inside the hole, a concentration cell forms which accelerates pitting.